
SL Paper 2
Consider the following list of organic compounds.
Compound 1: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\)
Compound 2: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
Compound 3: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
Compound 4: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\)
Hydrochloric acid neutralizes sodium hydroxide, forming sodium chloride and water.
\({\text{NaOH(aq)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\) \(\Delta {H^\Theta } = -57.9{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Apply IUPAC rules to state the name of compound 1.
(i) Define the term structural isomers.
(ii) Identify the two compounds in the list that are structural isomers of each other.
Determine the organic product formed when each of the compounds is heated under reflux with excess acidified potassium dichromate(VI). If no reaction occurs write NO REACTION in the table.
Explain the mechanism for the substitution reaction of bromoethane with sodium hydroxide. Use curly arrows to represent the movement of electron pairs.
(i) Define the term standard enthalpy change of reaction, \(\Delta {H^\Theta }\).
(ii) Determine the amount of energy released, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution reacts with \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid solution.
(iii) In an experiment, 2.50 g of solid sodium hydroxide was dissolved in \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. The temperature rose by 13.3 °C. Calculate the standard enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for dissolving one mole of solid sodium hydroxide in water.
\[{\text{NaOH(s)}} \to {\text{NaOH(aq)}}\]
(iv) Using relevant data from previous question parts, determine \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of solid sodium hydroxide with hydrochloric acid.
\[{\text{NaOH(s)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\]
\({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ethanoic acid were added to \({\text{30.0 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.150 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydrogencarbonate solution, \({\text{NaHC}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\).
The molar mass of a volatile organic liquid, X, can be determined experimentally by allowing it to vaporize completely at a controlled temperature and pressure. 0.348 g of X was injected into a gas syringe maintained at a temperature of 90 °C and a pressure of \(1.01 \times {10^5}{\text{ Pa}}\). Once it had reached equilibrium, the gas volume was measured as \({\text{95.0 c}}{{\text{m}}^{\text{3}}}\).
Bromoethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\), undergoes a substitution reaction to form ethanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\).
Outline how electrical conductivity can be used to distinguish between a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\), and a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of hydrochloric acid, HCl.
(i) State an equation for the reaction of ethanoic acid with a solution of sodium hydrogencarbonate.
(ii) Determine which is the limiting reagent. Show your working.
(iii) Calculate the mass, in g, of carbon dioxide produced.
(i) Determine the amount, in mol, of X in the gas syringe.
(ii) Calculate the molar mass of X.
(i) Identify the reagent necessary for this reaction to occur.
(ii) Deduce the mechanism for the reaction using equations and curly arrows to represent the movement of electron pairs.
Determine the enthalpy change, in kJ mol\(^{ - 1}\), for this reaction, using Table 10 of the Data Booklet.
Bromoethene, \({\text{C}}{{\text{H}}_{\text{2}}}{\text{CHBr}}\), can undergo polymerization. Draw a section of this polymer that contains six carbon atoms.
Ethene belongs to the homologous series of the alkenes.
A bromoalkane, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\), reacts with a warm, aqueous sodium hydroxide solution, NaOH.
The time taken to produce a certain amount of product using different initial concentrations of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) and NaOH is measured. The results are shown in the following table.
Outline three features of a homologous series.
Describe a test to distinguish ethene from ethane, including what is observed in each case.
Bromoethane can be produced either from ethene or from ethane. State an equation for each reaction.
State the equation for the reaction of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) with NaOH.
Suggest what would happen to the pH of the solution as the reaction proceeds.
Deduce the effect of the concentration of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) and NaOH on the rate of reaction.
C4H9Br:
NaOH:
Suggest why warm sodium hydroxide solution is used.
Deduce whether C4H9Br is a primary or tertiary halogenoalkane.
Determine the structural formula of C4H9Br.
Describe, using an equation, how \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) can be converted into \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{B}}{{\text{r}}_{\text{2}}}\).
Explain the mechanism for the reaction in (c) of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) with NaOH, using curly arrows to represent the movement of electron pairs.
In an experiment to measure the enthalpy change of combustion of ethanol, a student heated a copper calorimeter containing 100 cm3 of water with a spirit lamp and collected the following data.
\[\begin{array}{*{20}{l}} {{\text{Initial temperature of water:}}}&{{\text{20.0 }}^\circ {\text{C}}} \\ {{\text{Final temperature of water:}}}&{{\text{55.0 }}^\circ {\text{C}}} \\ {{\text{Mass of ethanol burned:}}}&{{\text{1.78 g}}} \\ {{\text{Density of water:}}}&{{\text{1.00 g}}\,{\text{c}}{{\text{m}}^{ - 3}}} \end{array}\]
(i) Use the data to calculate the heat evolved when the ethanol was combusted.
(ii) Calculate the enthalpy change of combustion per mole of ethanol.
(iii) Suggest two reasons why the result is not the same as the value in the Data Booklet.
Ethanol is part of the homologous series of alcohols. Describe two features of a homologous series.
(i) Below are four structural isomers of alcohols with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\). State the name of each of the isomers a, b, c and D.
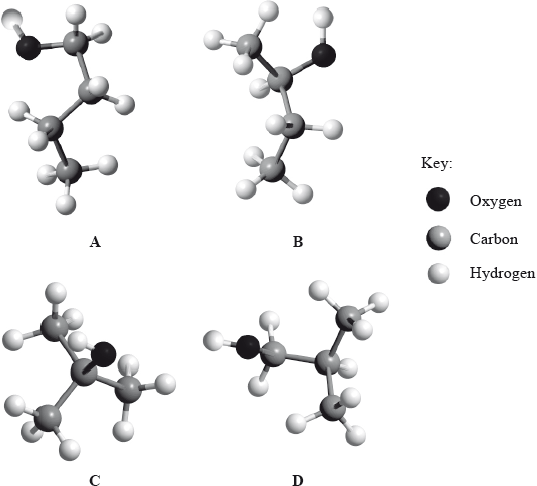
(ii) Determine the isomer that cannot be oxidized by acidifi ed potassium dichromate(VI), \({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\).
(iii) Determine the isomer which can be oxidized to butanal.
(iv) Determine the isomer which can be oxidized to butanone.
(v) Suggest the structural formula of another isomer of \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\).

(i) Isomer a is formed by reacting 1-bromobutane with aqueous sodium hydroxide. State whether the reaction would proceed via an SN1 or SN2 mechanism.
(ii) Explain the mechanism named in part (d) (i) using curly arrows to represent the movement of electron pairs.
Alkenes, alcohols and esters are three families of organic compounds with many commercial uses.
Esters are often used in perfumes. Analysis of a compound containing the ester functional group only, gives a percentage composition by mass of C: 62.0% and H: 10.4%.
State the meaning of the term structural isomers.
X is an isomer of C4H8 and has the structural formula shown below.
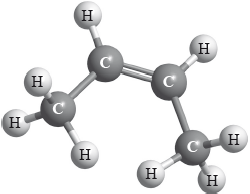
Apply IUPAC rules to name this isomer. Deduce the structural formulas of two other isomers of C4H8.
State the balanced chemical equation for the reaction of X with HBr to form Y.
Y reacts with aqueous sodium hydroxide, NaOH(aq), to form an alcohol, Z. Identify whether Z is a primary, secondary or tertiary alcohol.
Explain one suitable mechanism for the reaction in (v) using curly arrows to represent the movement of electron pairs.
Deduce the structural formula of the organic product formed when Z is oxidized by heating under reflux with acidified potassium dichromate(VI) and state the name of the functional group of this organic product.
Draw the ester functional group.
Determine the empirical formula of the ester, showing your working.
The molar mass of the ester is \({\text{116.18 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Determine its molecular formula.
Chloroethene, C2H3Cl, is an important organic compound used to manufacture the polymer poly(chloroethene).
Draw the Lewis structure for chloroethene and predict the H–C–Cl bond angle.
Draw a section of poly(chloroethene) containing six carbon atoms.
Outline why the polymerization of alkenes is of economic importance and why the disposal of plastics is a problem.
Chloroethene can be converted to ethanol in two steps. For each step deduce an overall equation for the reaction taking place.
Step 1:
Step 2:
State the reagents and conditions necessary to prepare ethanoic acid from ethanol in the laboratory.
State an equation, including state symbols, for the reaction of ethanoic acid with water. Identify a Brønsted-Lowry acid in the equation and its conjugate base.
Consider the following sequence of reactions.
\[{\text{RC}}{{\text{H}}_3}\xrightarrow{{reaction 1}}{\text{RC}}{{\text{H}}_2}{\text{Br}}\xrightarrow{{reaction 2}}{\text{RC}}{{\text{H}}_2}{\text{OH}}\xrightarrow{{reaction 3}}{\text{RCOOH}}\]
\({\text{RC}}{{\text{H}}_{\text{3}}}\) is an unknown alkane in which R represents an alkyl group.
The mechanism in reaction 2 is described as SN2.
Propan-1-ol has two structural isomers.
The alkane contains 81.7% by mass of carbon. Determine its empirical formula, showing your working.
Equal volumes of carbon dioxide and the unknown alkane are found to have the same mass, measured to an accuracy of two significant figures, at the same temperature and pressure. Deduce the molecular formula of the alkane.
(i) State the reagent and conditions needed for reaction 1.
(ii) State the reagent(s) and conditions needed for reaction 3.
Reaction 1 involves a free-radical mechanism. Describe the stepwise mechanism, by giving equations to represent the initiation, propagation and termination steps.
(i) State the meaning of each of the symbols in SN2.
(ii) Explain the mechanism of this reaction using curly arrows to show the movement of electron pairs, and draw the structure of the transition state.
(i) Deduce the structural formula of each isomer.
(ii) Identify the isomer from part (f) (i) which has the higher boiling point and explain your choice. Refer to both isomers in your explanation.
Alkenes, such as A (shown below), are important intermediates in the petrochemical industry because they undergo addition reactions to produce a wide variety of products, such as the conversion shown below.
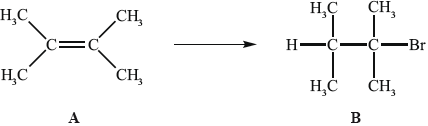
Another way to make B is the reaction shown below.
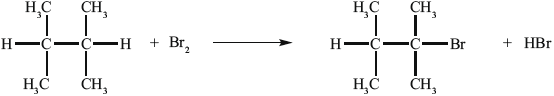
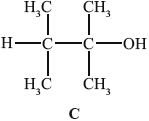
B can be converted into C.
In the gas phase, A reacts with hydrogen to form D.
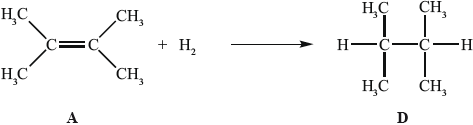
Applying IUPAC rules, state the name of A.
State the reagent required to convert A into B.
(i) State the conditions required for this reaction to occur.
(ii) Outline why it would give a poor yield of the desired product.
(i) State the reagent required.
(ii) Explain the mechanism of this reaction, using curly arrows to represent the movement of electron pairs.
A can also be converted into C without going via B. State the reagent and conditions required.
(i) State why C is not readily oxidized by acidified potassium dichromate(VI).
(ii) Deduce the structural formula of an isomer of C that could be oxidized to a carboxylic acid by this reagent.
State the conditions required for this reaction to occur.
State the homologous series to which D belongs.
Determine the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of A with hydrogen, using Table 10 of the Data Booklet, and state whether the reaction is exothermic or endothermic.
The standard enthalpy change of combustion of A is \( - 4000{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the amount of A, in mol, that would have to be burned to raise the temperature of \({\text{1 d}}{{\text{m}}^{\text{3}}}\) of water from 20 °C to 100 °C.